
 |
The Candela - unit of luminous intensity |
Light is that part of the spectrum of electromagnetic radiation that the human eye can see. It lies between about 400 and 700 nanometers. All the units for measuring and defining light are based on the candela, which is the unit defining the luminous intensity from a small source, in a particular direction. This unit used to be based on the light emission from a flame. Then the source became the glow from molten platinum. The current definition is a radical departure from the previous formulations, because it defines light intensity in terms of the unit for radiated power in general, the watt, or joule per second. The candela is therefore no longer strictly necessary as a fundamental unit, because it is defined in terms of another fundamental unit.
Historically, the engineers' unit of power, the watt, has been separated from the unit of luminous intensity, which is also a form of power, because the eye has a varying sensitivity over the visual spectrum, being relatively insensitive to blue and to red light. This radiation may make a deep impression on the viewer but, relative to yellow-green light, more watts of radiation are needed to cause a signal to reach the brain. Because of this the candela has to be defined for radiation at a single frequency. This makes the definition rather abstract, because no such light exists as something you can buy in a lamp store. The comforting symbolism of the candle has disappeared in the experts' merciless striving for scientific precision.
Here is the definition:
The candela is the luminous intensity, in a given direction, of a source that emits monochromatic radiation of frequency 540 × 1012 hertz and that has a radiant intensity in that direction of 1/683 watt per steradian.
Let us go through this definition bit by bit.
The frequency chosen is that to which the eye is most sensitive (more about this later). This frequency is normally quoted in the lighting literature as the corresponding wavelength: 555 nanometer. The wavelength varies with the medium through which the light passes, so, in the interest of precision, our relatively familiar wavelength description of light is not used in the standard.
The strange choice of the factor 683 is to make the value identical to that obtained with the previous version of the unit: the emission from 1 square centimeter of glowing, solidifying platinum.
The steradian is the cone of light spreading out from the source which would illuminate one square meter of the inner surface of a sphere of 1 m radius around the source.
The light intensity coming towards the observer is assumed to be reaching all angles within the enclosing steradian at the same intensity. It doesn't have to in practice: one can perfectly well measure the luminous intensity from a lighthouse beam, knowing that it actually only covers less than a hundredth of a steradian. One measures the light received by a small sensor of known area and multiplies this to give the corresponding value for one steradian
The definition implies a small source, because the energy stream from it is defined as energy within a given solid angle, independent of distance to the measuring instrument. If the source is very small, a tiny quartz halogen torch bulb for example, the brightness will appear to be intense even if its emission is one candela. If the source is, like a candle, small but not really a point, you will get an impression of a small area of light of moderate brightness, even though the light intensity is also one candela. The apparent brightness of a source when you look directly at it must not be confused with its luminous emission. The brightness of a source is measured in candela per square meter. Everything that is visible can be regarded as a light source. Museum objects become light sources when they reflect light from a lamp. We will return to the unit of brightness, the candela per square meter, later. It is an undeservedly neglected concept in exhibition design
There is no practical use in a museum for a light source giving monochromatic radiation at 540 × 1012 hz (555 nm wavelength in air), which the brain interprets as a yellow-green colour. This colour gives the best visibility per watt for an object such as black text on white paper. Good colour rendering, however, demands light sources that radiate over the whole visible spectrum. This inevitably reduces the efficiency of the lighting, because the eye needs more energy in the red and blue-violet ends of the spectrum to give the same visual clarity.
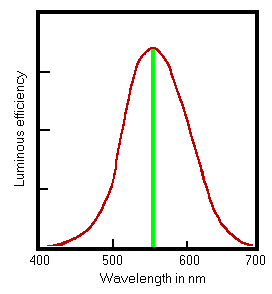 The measurement of luminous intensity from a useful light source requires extra information: the relative sensitivity of the eye to different wavelengths. The luminous intensity of a "white" light source is defined by multiplying the watts emitted at each wavelength by the efficiency of that wavelength in exciting the eye, relative to the efficiency at 555 nm. This efficiency factor goes under the curious name of the V-lambda curve. Here it is.
The measurement of luminous intensity from a useful light source requires extra information: the relative sensitivity of the eye to different wavelengths. The luminous intensity of a "white" light source is defined by multiplying the watts emitted at each wavelength by the efficiency of that wavelength in exciting the eye, relative to the efficiency at 555 nm. This efficiency factor goes under the curious name of the V-lambda curve. Here it is.
This curve, obtained by averaging results from experiments with many people, has long been standardised as an essential component in the quantitative description of light. The curve defines the relationship between the human sensation of light and the physical concept of energy, which is the quantity which measuring instruments react to.
The watts emitted by a light source can be measured by absorbing all the light in a perfectly black surface and measuring the heat produced. A filter corresponding to the V-lambda curve can be placed in front of the black absorber to convert the result to what the human eye and brain regard as 'brightness'.
Practical measuring instruments contain semiconductor sensors which convert the absorbed light into electric current.

 The efficient lighting provided by monochromatic radiation around 555nm is too weird to be acceptable even to office workers. Fluorescent lamps for office use
are therefore cunningly designed to have a large emission in the central region of
the spectrum, where one gets the most visual clarity for the watts driven
throught the fluorescent tube, with a bit of blue and red added to fool the
brain into thinking that the light is white. The picture to the left has
yellow-green stripes interlaced with grey. The picture on the right has
the same yellow-green stripes interlaced with red and blue. The partial
whitening of the strong green impression is best observed from a distance.
The efficient lighting provided by monochromatic radiation around 555nm is too weird to be acceptable even to office workers. Fluorescent lamps for office use
are therefore cunningly designed to have a large emission in the central region of
the spectrum, where one gets the most visual clarity for the watts driven
throught the fluorescent tube, with a bit of blue and red added to fool the
brain into thinking that the light is white. The picture to the left has
yellow-green stripes interlaced with grey. The picture on the right has
the same yellow-green stripes interlaced with red and blue. The partial
whitening of the strong green impression is best observed from a distance.
If you are unimpressed by this demonstration, which depends somewhat
on your computer, there is another way of making visible the strong green
emission of office lighting.  Take
a compact disk and hold it under, but at a distance of several meters
from, a fluorescent tube. Even better, make a small slit in a piece of
cardboard to reduce the light from the tube to a parallel stream from a
small source. Wiggle the disk around (music side towards you). In addition to the direct reflection of the light source
you will pick up a diffracted image in which the light is separated into
coloured bands. Notice the strong blue bands, that come from the mercury
vapour in the tube, the intense green emission, from the main phosphor,
and a red emission from another phosphor.
Take
a compact disk and hold it under, but at a distance of several meters
from, a fluorescent tube. Even better, make a small slit in a piece of
cardboard to reduce the light from the tube to a parallel stream from a
small source. Wiggle the disk around (music side towards you). In addition to the direct reflection of the light source
you will pick up a diffracted image in which the light is separated into
coloured bands. Notice the strong blue bands, that come from the mercury
vapour in the tube, the intense green emission, from the main phosphor,
and a red emission from another phosphor.
Compare this with the continuous spectra from daylight and from incandescent lamps.
This excess of green radiation in office lighting is why photographs taken in offices usually have a greenish cast.
Fluorescent lamps are also made with a more even spread of energy through the spectrum but the energy efficiency is lower. The eye cannot tell the difference between these two lights by looking at them but does notice the bad colour rendering when looking at objects under the efficient light. Measuring this colour rendering, and deciding how much it matters, is the subject of a later article.
Now that I have explained the candela in detail, I will just mention that although it is fundamental to all other units of light, you won't often meet it. What matters to the architect and engineer is the total light emission of a lamp and its distribution in space. What matters to the conservator is the light falling on the object, usually from several light sources. This requires the introduction of the lumen and the lux. Next time.

This work is licensed under a Creative Commons Attribution-Noncommercial-No Derivative Works 3.0 License.